ACUTE MYELOID LEUKEMIA (AML) IS A HETEROGENEOUS HEMATOLOGIC MALIGNANCY1
Approximately 80% of AML patient samples overexpressed a pro-survival BCL-2 family protein in a post hoc translational study2-4
Approximately 80% of AML patient samples overexpressed a pro-survival BCL-2 family protein in a post hoc translational study2-4
Overexpression of pro-survival BCL-2 family regulator proteins is an important driver of resistance to cell death in a heterogeneous disease4
PRO-SURVIVAL REGULATOR PROTEINS
Some include BCL-2, BCL-XL, and MCL-1, all of which play a key role in regulating apoptosis.2,4
IN A STUDY OF 1540 AML PATIENTS3
driver
mutations
mutations
genes or
genomic regions
genomic regions
of patients had
≥ 2 mutations
≥ 2 mutations
Clonal evolution within each AML patient is a dynamic process in which there is continuous acquisition and loss of specific mutations, often occurring at different time points over the course of the disease.5,6 AML is characterized by clonal heterogeneity and ongoing clonal evolution that may influence the selection of clones. This makes the AML genome a moving target over time.6
Overexpression of pro-survival BCL-2 family proteins inhibits apoptosis and is a common survival mechanism employed by leukemic cells7
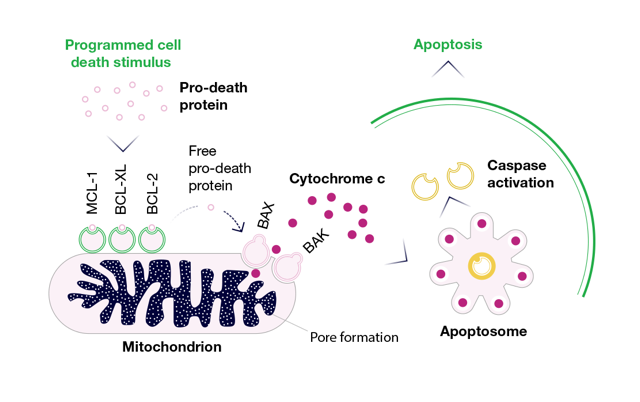
THE BCL-2 FAMILY ACTS AS AN APOPTOTIC SWITCH FOR INTRINSIC MITOCHONDRIAL-MEDIATED CELL DEATH4,8
The BCL-2 family consists of pro-survival and pro-death proteins that function cooperatively to regulate the intrinsic pathway of apoptosis.
Some, such as MCL-1, BCL-XL, and BCL-2, block cell death by sequestering and preventing the activation of pro-death proteins, leading to cancer cell survival. Other members of the BCL-2 family are pro-apoptotic, such as BAX, BAK, and BOK, and mediate mitochondrial membrane permeabilization, a critical event that leads to programmed cell death.4,8
Pro-survival BCL-2 family overexpression in AML is a common feature in a complex, heterogeneous disorder.1,4,5
THE BCL-2 Family Regulator Proteins Are KEY TO APOPTOSIS4,8
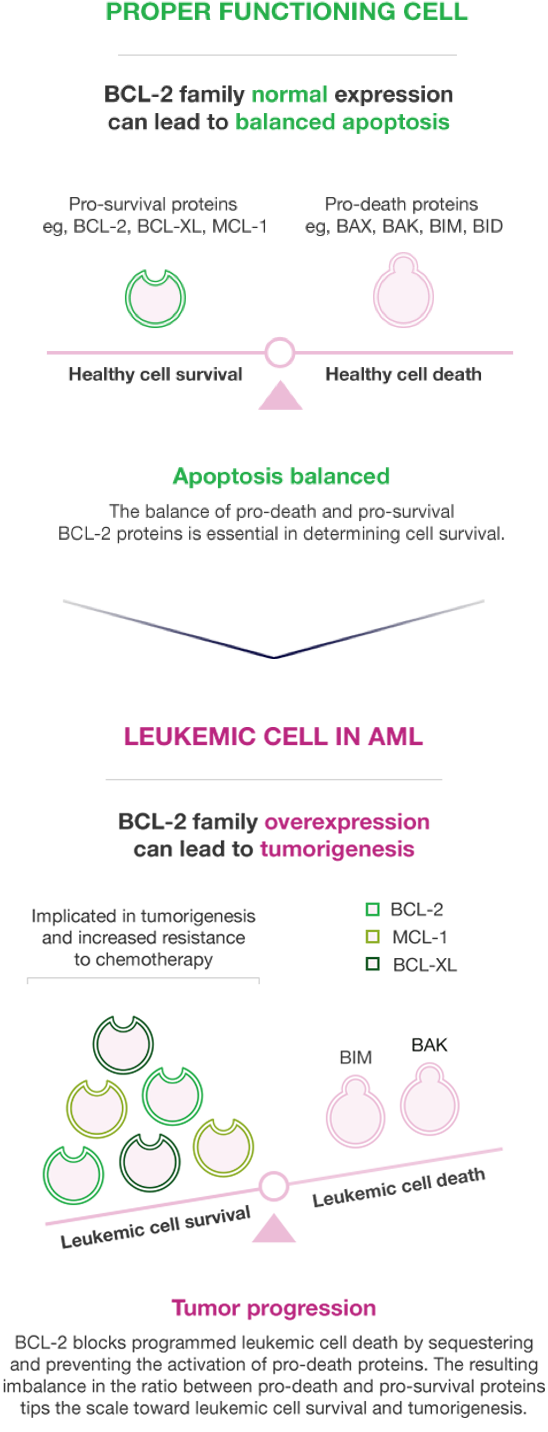
DYSREGULATION OF THE APOPTOTIC PATHWAY CAN SHIFT LEUKEMIC CELLS TO A PRO-SURVIVAL MODE4,7-10
DYSREGULATION OF THE APOPTOTIC PATHWAY CAN SHIFT LEUKEMIC CELLS TO A PRO-SURVIVAL MODE4,7-10
Malignant cell survival is based on the relative ratio of pro-survival proteins, such as BCL-2, BCL-XL, and MCL-1, and pro-death proteins, such as BAX, BIM, and BAK7,9,10
Shifting the ratio of BCL-2 pro-survival and pro-death proteins is important to AML's pathophysiology.4,8
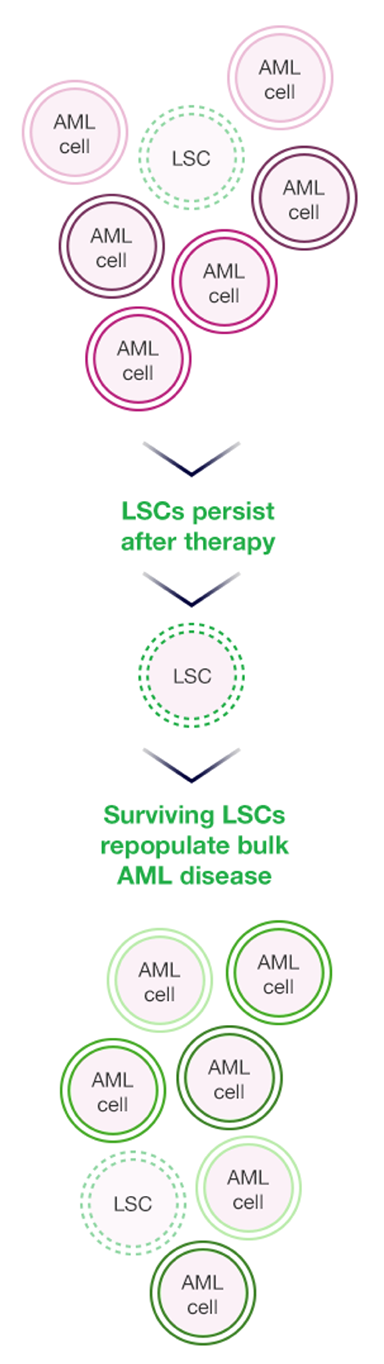
THE ROLE OF BCL-2 FAMILY REGULATOR PROTEINS IN LEUKEMIC STEM CELLS (LSCs) IS EMERGING13,14
Historically, the continued persistence of AML has been attributed to the mutational profile of the disease. However, new data have emerged suggesting that preexisting therapy-resistant leukemia stem cell populations perpetuate the disease and drive disease relapse.15 Interestingly, certain members of the BCL-2 family can be overexpressed in quiescent leukemia stem cells and may represent an important driver of chemoresistance.9
THERAPY-RESISTANT LSCs PERPETUATE DISEASE AND DRIVE RELAPSE9,16
THERAPY-RESISTANT LSCs PERPETUATE DISEASE AND DRIVE RELAPSE9,16
Stay tuned for research updates on the role of BCL-2 family regulator proteins in targeting LSCs.
THE LANDSCAPE IN AML IS CHANGING17
For decades, there have been limited changes in the management of AML, and an unmet need still remains.17 Intensive chemotherapy remains the standard of care in AML, with approximately 70% of younger patients and 50% of older patients achieving CR after one or two induction treatments. However, patient age may affect the ability to tolerate intensive treatment.18
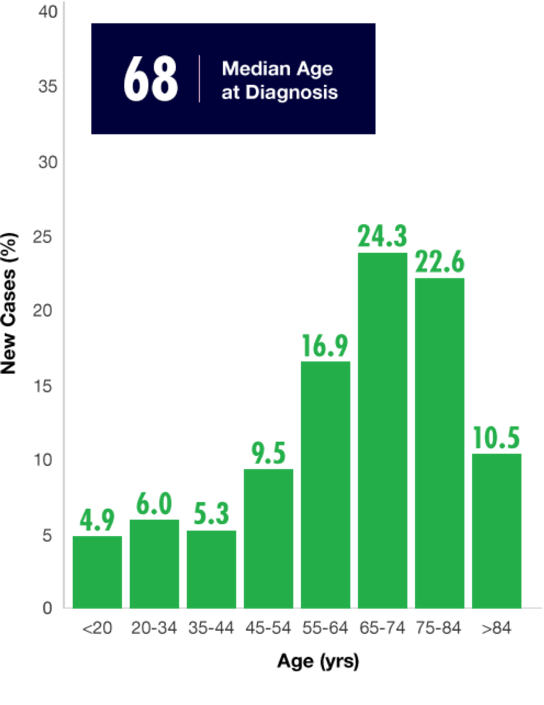
With a median patient age of 68, AML is primarily a disease of older adults who generally have a poor prognosis.19,20 Prognosis is influenced by individual patient characteristics such as age, presence of comorbid conditions affecting performance status, and preexisting myelodysplasia. This is particularly true of elderly patients with AML.18,20,21
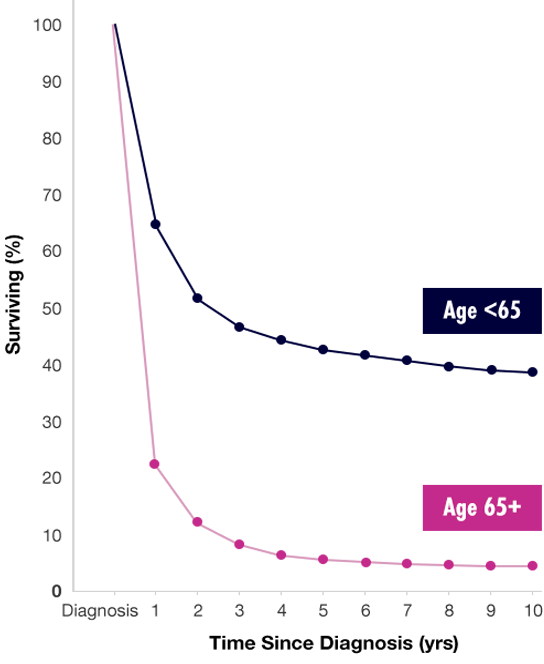
Survival rates are lowest in populations with the highest prevalence19,22
Survival rates are lowest in populations with the highest prevalence19,22
Common factors affecting prognosis
Common factors affecting prognosis
AbbVie is committed to ongoing research in AML.
BCL-2=B-cell lymphoma 2; CCI=Charlson Comorbidity Index; CR=complete response; ECOG=Eastern Cooperative Oncology Group; FLT=fms-like tyrosine kinase; IDH=isocitrate dehydrogenase.
References:
Li S, Garrett-Bakelman FE, Chung SS, et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016;22(7):792-799.
Banker DE, Groudine M, Norwood T, Appelbaum FR. Measurement of spontaneous and therapeutic agent-induced apoptosis with BCL-2 protein expression in acute myeloid leukemia. Blood. 1997; 89(1):243-255.
Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221.
Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb). 2011;3(4):279-296.
Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510.
Paguirigan A, Smith J, Meshinchi S, et al. Single-cell genotyping demonstrates complex clonal diversity in acute myeloid leukemia. Sci Transl Med. 2015;7(281):1-18.
Vo TT, Ryan J, Carrasco R, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151(2):344-355.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70.
Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329-341.
Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15(5):1132-1144.
Chan SM, Thomas D, Corces-Zimmerman MR, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21(2):178-184.
Irish JM, Ånensen N, Hovland R, et al. Flt3 Y591 duplication and Bcl-2 overexpression are detected in acute myeloid leukemia cells with high levels of phosphorylated wild-type p53. Blood. 2007;109(6):2589-2596.
Pollyea DA, Jordan CT. Therapeutic targeting of acute myeloid leukemia stem cells. Blood. 2017;129(12):1627-1635.
Moujalled DM, Pomillo G, Ghiurau C, et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. [published online ahead of print September 10, 2018]. Leukemia. doi:10.1038/s41375-018-0261-3.
Stahl M, Kim TK, Zeidan AM. Update on acute myeloid leukemia stem cells: new discoveries and therapeutic opportunities. World J Stem Cells. 2016;8(10):316-331.
Felipe Rico J, Hassane DC, Guzman ML. Acute myelogenous leukemia stem cells: from bench to bedside. Cancer Lett. 2013;338(1):4-9.
Perl AE. The role of targeted therapy in the management of patients with AML. Blood Adv. 2017;1(24):2281-2294.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V3.2018. National Comprehensive Cancer Network, Inc. 2018. All rights reserved. Accessed December 5, 2018. To view the most recent and complete version of the guideline, go online to NCCN.org.
National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: leukemia—acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html. Accessed November 6, 2018.
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152.
Yogarajah M, Stone RM. A concise review of BCL-2 inhibition in acute myeloid leukemia. Expert Rev Hematol. 2018;11(2):145-154.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: SEER 9. Acute Myeloid Leukemia. SEER Survival Rates by Time Since Diagnosis, 2000-2014, by Age. Released November 2018.
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655.
ECOG-ACRIN Cancer Research Group. ECOG performance status. https://ecog-acrin.org/resources/ecog-performance-status. Accessed October 22, 2018.
Klepin HD. Elderly acute myeloid leukemia: assessing risk. Current Hematol Malig Rep. 2015;10(2):118-125.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.